Products In A Chemical Equation
Products are the species formed from chemic reactions.[1] During a chemic reaction, reactants are transformed into products after passing through a high energy transition country. This process results in the consumption of the reactants. Information technology can exist a spontaneous reaction or mediated by catalysts which lower the energy of the transition state, and by solvents which provide the chemic environment necessary for the reaction to take place. When represented in chemic equations, products are by convention drawn on the right-hand side, even in the case of reversible reactions.[ii] The backdrop of products such every bit their energies help decide several characteristics of a chemic reaction, such equally whether the reaction is exergonic or endergonic. Additionally, the backdrop of a product can make information technology easier to extract and purify following a chemic reaction, peculiarly if the product has a different state of matter than the reactants.
| Spontaneous reaction
| Catalysed reaction
|
Much of chemical science research is focused on the synthesis and characterization of benign products, as well as the detection and removal of undesirable products. Synthetic chemists tin be subdivided into enquiry chemists who design new chemicals and pioneer new methods for synthesizing chemicals, as well every bit process chemists who calibration up chemic production and go far safer, more environmentally sustainable, and more efficient.[iii] Other fields include natural product chemists who isolate products created by living organisms then characterize and study these products.
Determination of reaction [edit]
The products of a chemical reaction influence several aspects of the reaction. If the products are lower in energy than the reactants, then the reaction will give off backlog energy making it an exergonic reaction. Such reactions are thermodynamically favorable and tend to happen on their own. If the kinetics of the reaction are high enough, all the same, then the reaction may occur as well slowly to exist observed, or not even occur at all. This is the example with the conversion of diamond to lower energy graphite at atmospheric pressure level, in such a reaction diamond is considered metastable and will not exist observed converting into graphite.[4] [five]
If the products are higher in chemical energy than the reactants then the reaction volition require free energy to exist performed and is therefore an endergonic reaction. Additionally if the product is less stable than a reactant, then Leffler'south supposition holds that the transition state will more closely resemble the product than the reactant.[6] Sometimes the product will differ significantly plenty from the reactant that information technology is easily purified post-obit the reaction such as when a production is insoluble and precipitates out of solution while the reactants remained dissolved.
History [edit]
Ever since the mid-nineteenth century, chemists have been increasingly preoccupied with synthesizing chemical products.[vii] Disciplines focused on isolation and characterization of products, such as natural products chemists, remain important to the field, and the combination of their contributions alongside constructed chemists has resulted in much of the framework through which chemical science is understood today.[7]
Much of synthetic chemistry is concerned with the synthesis of new chemicals as occurs in the design and creation of new drugs, likewise as the discovery of new synthetic techniques. Get-go in the early 2000s, process chemistry began emerging as a distinct field of synthetic chemistry focused on scaling up chemical synthesis to industrial levels, as well as finding ways to make these processes more than efficient, safer, and environmentally responsible.[3]
Biochemistry [edit]
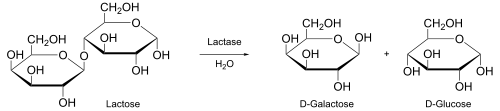
In biochemistry, enzymes human activity as biological catalysts to convert substrate to product.[viii] For example, the products of the enzyme lactase are galactose and glucose, which are produced from the substrate lactose.
- Where South is substrate, P is production and E is enzyme.
Production promiscuity [edit]
Some enzymes display a course of promiscuity where they convert a single substrate into multiple dissimilar products. Information technology occurs when the reaction occurs via a loftier free energy transition state that tin can be resolved into a diversity of different chemic products.[9]
Product inhibition [edit]
Some enzymes are inhibited by the production of their reaction binds to the enzyme and reduces its activity.[10] This can be of import in the regulation of metabolism equally a form of negative feedback controlling metabolic pathways.[11] Production inhibition is also an important topic in biotechnology, as overcoming this consequence can increase the yield of a product.[12]
Come across too [edit]
- Chemical reaction
- Substrate
- Reagent
- Precursor
- Catalyst
- Enzyme
- Production
- Derivative
- Chemic equilibrium
- 2d constabulary of thermodynamics
References [edit]
- ^ McNaught, A. D.; Wilkinson, A. (2006). [production] Compendium of Chemical Terminology, 2nd ed. (the "Golden Volume". Blackwell Scientific Publications, Oxford. doi:10.1351/goldbook. ISBN978-0-9678550-nine-seven.
- ^ McNaught, A. D.; Wilkinson, A. (2006). [chemical reaction equation] Compendium of Chemic Terminology, second ed. (the "Gold Book"). Blackwell Scientific Publications, Oxford. doi:10.1351/goldbook. ISBN978-0-9678550-9-7.
- ^ a b Henry, Celia M. "DRUG DEVELOPMENT". Chemical and Engineering News. Retrieved xiii September 2014.
- ^ McNaught, A. D.; Wilkinson, A. (2006). [diamond] Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Blackwell Scientific Publications, Oxford. doi:10.1351/goldbook. ISBN978-0-9678550-9-7.
- ^ McNaught, A. D.; Wilkinson, A. (2006). [metastability] Compendium of Chemical Terminology, second ed. (the "Gold Book"). Blackwell Scientific Publications, Oxford. doi:10.1351/goldbook. ISBN978-0-9678550-nine-seven.
- ^ McNaught, A. D.; Wilkinson, A. (2006). [metastability] Compendium of Chemical Terminology, 2nd ed. (the "Gold Volume"). Blackwell Scientific Publications, Oxford. doi:10.1351/goldbook. ISBN978-0-9678550-9-seven.
- ^ a b Yeh, Brian J; Lim, Wendell A (2007). "Synthetic biology: lessons from the history of constructed organic chemistry". Nature Chemical Biology. iii (9): 521–525. doi:ten.1038/nchembio0907-521. PMID 17710092. S2CID 17719341.
- ^ Cornish-Bowden, A (2 September 2013). "The origins of enzyme kinetics". FEBS Letters. 587 (17): 2725–xxx. doi:x.1016/j.febslet.2013.06.009. PMID 23791665. S2CID 12573784.
- ^ Yoshikuni, Y; Ferrin, TE; Keasling, JD (20 Apr 2006). "Designed divergent evolution of enzyme office". Nature. 440 (7087): 1078–82. Bibcode:2006Natur.440.1078Y. doi:x.1038/nature04607. PMID 16495946. S2CID 4394693.
- ^ Walter C, Frieden E (1963). "The prevalence and significance of the product inhibition of enzymes". Advances in Enzymology and Related Areas of Molecular Biology. Adv. Enzymol. Relat. Areas Mol. Biol. Advances in Enzymology - and Related Areas of Molecular Biological science. Vol. 25. pp. 167–274. doi:10.1002/9780470122709.ch4. ISBN978-0-470-12270-nine. PMID 14149677.
- ^ Hutson NJ, Kerbey AL, Randle PJ, Sugden PH (1979). "Regulation of pyruvate dehydrogenase by insulin action". Prog. Clin. Biol. Res. 31: 707–xix. PMID 231784.
- ^ Schügerl K, Hubbuch J (2005). "Integrated bioprocesses". Curr. Opin. Microbiol. 8 (3): 294–300. doi:x.1016/j.mib.2005.01.002. PMID 15939352.
Products In A Chemical Equation,
Source: https://en.wikipedia.org/wiki/Product_(chemistry)
Posted by: sosaammed1971.blogspot.com





0 Response to "Products In A Chemical Equation"
Post a Comment